Multiple Choice
Assume that only two isotopes of argon, 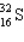 and
and 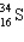 exist in nature. If this were true, the atomic weight of sulfur would fall most closely within which range?
exist in nature. If this were true, the atomic weight of sulfur would fall most closely within which range?
A) 16-33.5 amu
B) 16-34 amu
C) 16-50 amu
D) 32-34 amu
E) 48-50 amu
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Suppose the isotopic ratio of the two
Q3: Which element has an atomic number of
Q4: Three elements that are likely to have
Q5: Write the atomic symbol for an atom
Q6: Discuss what role Rutherford's gold foil experiment
Q8: Which sample contains the same number of
Q9: How many millimeters are in 0.457 yards?<br>A)
Q10: Which statement best describes isotopes?<br>A) atoms in
Q11: Which element can be classified as a
Q12: Which of the following is the most