Multiple Choice
Exhibit 18-2 Use this list of half-reactions to answer the following question(s) .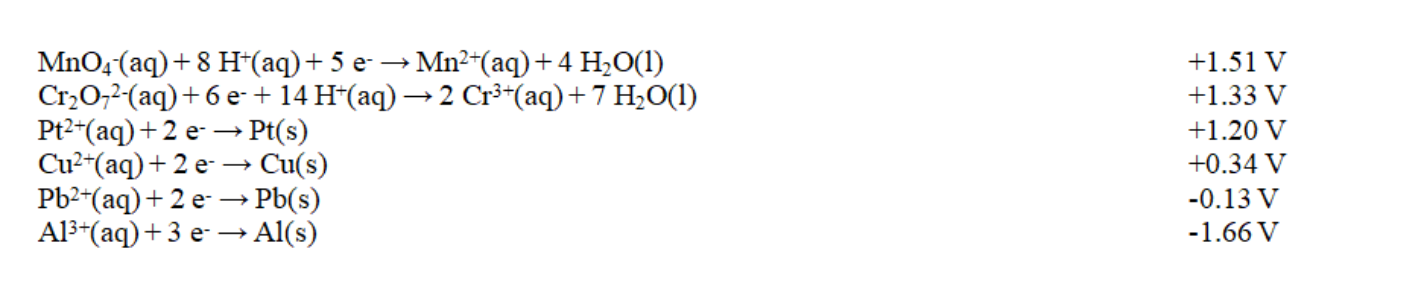 Refer to Exhibit 18-2. The potential for the product-favored reaction involving aluminum and copper metals, Al3+(aq) and Cu2+(aq) , is
Refer to Exhibit 18-2. The potential for the product-favored reaction involving aluminum and copper metals, Al3+(aq) and Cu2+(aq) , is
A) 2.17 V.
B) 2.00 V.
C) 1.79 V.
D) 1.32 V.
E) 1.15 V.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: The study of the relationships between electron
Q42: In the reaction shown below, _ is
Q43: Calculate the value of E<sub>cell</sub> for
Q44: A fuel cell is<br>A) an electrolytic cell
Q45: Exhibit 18-2 Use this list of half-reactions
Q47: The relationship between Gibbs free energy
Q48: Explain, with reference to the Nernst equation,
Q49: Balance the following redox equation.<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg"
Q50: The value of E<sup> <span class="ql-formula"
Q51: A secondary cell is one which _;