Multiple Choice
Exhibit 18-2 Use this list of half-reactions to answer the following question(s) .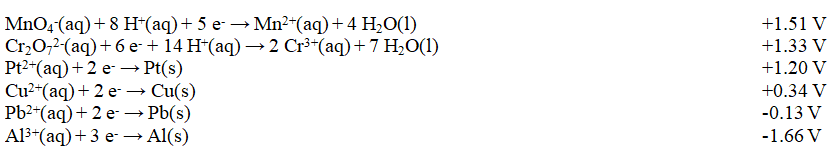 Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
Refer to Exhibit 18-2. Which of these combinations would result in a spontaneous reaction?
A) Al3+(aq) and Cr3+(aq)
B) Al3+(aq) and Cu(s)
C) Cr2O72-(aq) and MnO4-(aq)
D) Cu(s) and MnO4-(aq)
E) Pt(s) and Pb2+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Refer to the following values of standard
Q17: Which statement concerning the proton exchange membrane
Q18: Which change does not indicate a reduction?<br>A)
Q19: In the salt bridge in an electrochemical
Q20: If cadmium metal and the Fe(III) ion
Q22: Calculate the time needed to plate out
Q23: Which cell notation represents a battery constructed
Q24: The unit used to measure electromotive force
Q25: Is the part of a flashlight battery
Q26: Which of the following would require the