Multiple Choice
Calculate the value of DS for the reaction shown: 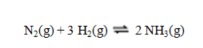 At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
At 25 C the values of entropy in J K-1 mol-1 are nitrogen, 191.61: hydrogen, 130.68; and ammonia, 192.77.
A) -198.11 J/K
B) -259.03 J/K
C) -390.88 J/K
D) -393.20 J/K
E) -969.19 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: A reaction is exothermic and has
Q31: At constant T and P, in which
Q32: Use the data given to calculate
Q33: For a specific chemical reaction, the
Q34: Five coins are tossed. Which combination of
Q36: The value of DG<sup> <span class="ql-formula"
Q37: Two substances, A and B, have
Q38: How many of the following processes involve
Q39: At a particular temperature, a reactant-favored process
Q40: A reaction cannot change between being