Multiple Choice
Use the data given to calculate the value of DG rxn at 25 C for the reaction given below. DG f for CO(g) is -137.16 kJ/mol. 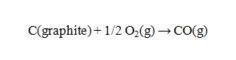
A) -274.32 kJ
B) -137.16 kJ
C) -68.58 kJ
D) +137.16 kJ
E) +274.32 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Which process involves a decrease of entropy
Q21: If a reaction is product-favored at
Q22: Consider the options given for the distribution
Q23: Which statement is not correct?<br>A) the
Q24: Since no chemical process is 100% efficient,
Q26: For a particular reaction, the value
Q27: Compression of a gas is<br>A) reactant-favored, since
Q28: Which statement is correct?<br>A) The entropy of
Q29: DS<sup> <span class="ql-formula" data-value="\circ"><span class="katex"><span class="katex-mathml"><math
Q30: A reaction is exothermic and has