Multiple Choice
Use the data at 25 C given below to calculate the value of DG rxn for the reaction shown when it takes place at 120 C. 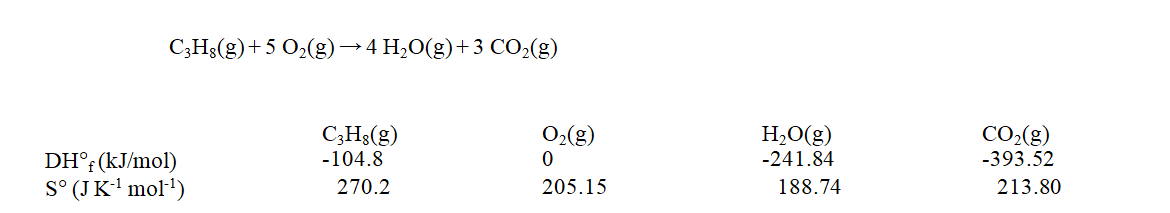
A) -2004 kJ
B) -2046 kJ
C) -2055 kJ
D) -2073 kJ
E) -2083 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: If a chemical reaction is at
Q10: Which set of conditions describes a reaction
Q11: Which statement correctly describes the meaning
Q12: Which has the highest entropy at a
Q13: The enthalpy change for a reaction
Q15: A certain reaction has DH<sup> <span
Q16: Which process is reactant-favored?<br>A) decomposition of
Q17: At constant T and P, in which
Q18: Nature favors exothermic reactions because after such
Q19: What is the value of the