Multiple Choice
Use the data given to calculate the value of K for the reaction at 25 C. 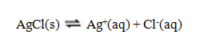

A) 6.61 × 10-14
B) 3.41 × 10-12
C) 4.75 × 10-12
D) 1.76 × 10-10
E) 5.69 × 109
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q39: At a particular temperature, a reactant-favored process
Q40: A reaction cannot change between being
Q41: If two reactions are coupled, then<br>A) A
Q42: Which set of conditions describes a reaction
Q43: The value of the equilibrium constant
Q45: Which statement is false?<br>A) energy will disperse
Q46: A reaction is product-favored when<br>A) K<sup>
Q47: It is possible for a substance, with
Q48: A slight change in temperature can
Q49: At constant T and P, in which