Multiple Choice
In this reaction 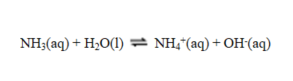
A) NH3 acts as a base and OH- as an acid.
B) H2O acts as an acid and NH3 as a base.
C) H2O acts as a base and NH4+ as an acid.
D) H2O acts as an acid and NH4+ as a base.
E) NH3 acts as an acid and OH- as a base.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: The value of the ionization constant for
Q12: The pH of a 0.172 M solution
Q13: The reaction between the ion of a
Q14: Which compound is the most acidic?<br>A) GeH<sub>4</sub><br>B)
Q15: Which reaction illustrates water acting as a
Q17: Answer the following questions:<br>A) Write the reaction for
Q18: The pH of a solution of a
Q19: The pH of a 0.50 M solution
Q20: As the pH of a solution rises,
Q21: Which pair of substances does not represent