Multiple Choice
The conjugate base of NH3 is ____.
A) H2O
B) OH-
C) 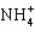
D) NH4OH
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Which factor does not contribute to the
Q31: Which statement is not correct?<br>A) a Lewis
Q32: Amines have the general formula<br>A) R-CHO<br>B) CH<sub>3</sub>-(CH<sub>2</sub>)<sub>n</sub>-CH<sub>3</sub><br>C)
Q33: Calculate the pH of a 0.051 M
Q34: Which of the following salts forms a
Q36: Bronsted-Lowry acids _ and Bronsted-Lowry bases _.<br>A)
Q37: Which substance can act as a Bronsted-Lowry
Q38: Which salt forms a neutral solution when
Q39: Because water can act as a Bronsted-Lowry
Q40: Classify each compound as a carboxylic acid