Multiple Choice
Arrange the solutions in order of increasing acidity: 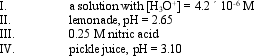
A) I < IV < II < III
B) II < IV < III < I
C) III < II < IV < I
D) IV < I < II < III
E) III < II < I < IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: The acid ionization constant expression for the
Q43: In a 1.2 M solution of HClO<sub>4</sub>,
Q44: Identify which of the hydrohalic acids is
Q45: For the dissociation of a very weak
Q46: Ammonia is a weak base and perchloric
Q48: Ammonia is a weak base and acetic
Q49: Which pair of substances represents a conjugate
Q50: Typically, for a weak acid and its
Q51: In a basic solution at 25<sup>
Q52: A Bronsted-Lowry acid is always<br>A) a highly