Multiple Choice
Consider the equilibrium reaction 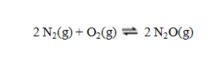 In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
In a particular experiment, the equilibrium concentration of N2 is 0.048 M, of O2, 0.093 M, and of N2O, 6.55 × 10-21 M. What is the value of the equilibrium constant Kc?
A) 1.5 × 10-18
B) 2.0 × 10-37
C) 2.2 × 10-36
D) 3.1 × 10-17
E) 5.0 × 1036
Correct Answer:

Verified
Correct Answer:
Verified
Q4: As the temperature is increased, which kinds
Q5: An endothermic reaction which results in an
Q6: If the value of K<sub>c</sub> for a
Q7: To decide whether a reaction mixture is
Q8: Terms relating to certain types of substances
Q10: The equilibrium constant for the reaction <img
Q11: Consider the exothermic reaction at equilibrium: <img
Q12: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the
Q13: Considering only the probability factor in the
Q14: The concentration equilibrium constant, K<sub>c</sub>, and the