Multiple Choice
For the reaction 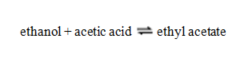 Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
Kc = 0.95. A mixture of the three substances contains 0.45 M ethanol, 0.45 M acetic acid and 1.1 M ethyl acetate. Which statement is true?
A) Q < K, so the system will react left to right.
B) Q < K, so the system will react right to left.
C) The mixture is at equilibrium.
D) Q > K, so the system will react left to right.
E) Q > K, so the system will react right to left.
Correct Answer:

Verified
Correct Answer:
Verified
Q35: A weak acid is 5% ionized at
Q36: A chemical reaction reaches equilibrium when<br>A) both
Q37: The equilibrium constant for a particular chemical
Q38: Consider the reaction<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="
Q39: A particular chemical reaction is<br>A) reactant-favored if
Q41: Consider the equilibrium system <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q42: In a balanced chemical equation, if there
Q43: If the reaction quotient, Q, is greater
Q44: Which of the following is not true
Q45: For an exothermic reaction, an increase in