Multiple Choice
From the stoichiometry of the reaction 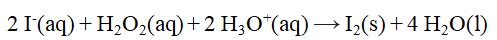 the rate law
the rate law
A) is predicted to be third-order overall.
B) is predicted to be first-order in I2(s) .
C) is predicted to be first-order in all reactants.
D) is predicted to be fifth-order overall.
E) cannot be predicted.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: A reaction displays zero-order kinetics for its
Q25: If an elementary reaction is exothermic, then<br>A)
Q26: The rate constant for the reaction
Q27: Consider the hypothetical reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider
Q28: For a new chemical reaction involving several
Q30: In a bimolecular reaction, which term can
Q31: Which of the following could not be
Q32: Which statement is true about a catalyst?<br>A)
Q33: The species representing the combination of atoms
Q34: When two atoms, molecules or ions are