Multiple Choice
The following is the reaction that occurs in automobile airbags: 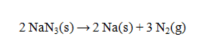 How many grams of sodium azide (NaN3) are required to produce 19.00 L of N2 at 293K and 775 mm Hg? R = 0.0821 L atm mol-1 K-1.
How many grams of sodium azide (NaN3) are required to produce 19.00 L of N2 at 293K and 775 mm Hg? R = 0.0821 L atm mol-1 K-1.
A) 34.9 g
B) 52.0 g
C) 2.66 × 104 g
D) 3.89 × 105 g
E) 5.80 × 105 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Determine the volume (in L) of Cl<sub>2</sub>(g)
Q27: For a gas mixture<br>A) the partial pressures
Q28: A 5.00 L flask contains 3.50 g
Q29: Under what conditions do real gases most
Q30: Suppose that 1.00 gram of each of
Q32: Which is not a greenhouse gas?<br>A) carbon
Q33: If 7.63 g of an unknown gas
Q34: If a 2.0-liter sample of gas experiences
Q35: Which two assumptions of the Kinetic-Molecular Theory
Q36: Which description of ideal gases and real