Multiple Choice
What volume (in L) of oxygen at 298K and 1.50 atm is required for the complete combustion of 25.0 g of hexane? The value of R = 0.0821 L atm mol-1 K-1. 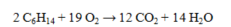
A) 4.73 L
B) 0.397 L
C) 34.2 L
D) 45.0 L
E) 407 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q47: What is the pressure of 2.50 mol
Q48: Suppose that in a known volume of
Q49: How are the pressure and the absolute
Q50: Suppose that at STP a gas occupies
Q51: Which statement about ideal gases and the
Q53: If at 29°C and 2.50 atm of
Q54: Hydrochloric acid reacts with magnesium to produce
Q55: Clean air<br>A) is pure oxygen.<br>B) contains mostly
Q56: What is the density (in g/L) of
Q57: Which is the most abundant gas found