Multiple Choice
Solid potassium chlorate can be decomposed to produce potassium chloride and oxygen according to the following equation. 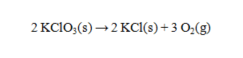 How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
How many grams of KClO3 are needed to produce 752 mL of oxygen at 23°C and 0.995 atm, if the oxygen is collected over water? The vapor pressure of water at this temperature is 21.07 torr.
A) 0.0308 g
B) 2.43 g
C) 2.52 g
D) 3.66 g
E) 3.77 g
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What is the density of oxygen gas
Q16: Which is a mathematical representation of Avogadro's
Q17: What is the correct name for the
Q18: A mixture of H<sub>2</sub>(g) and Cl<sub>2</sub>(g) exerts
Q19: Which description of the kinetic-molecular theory of
Q21: What is the molar mass of an
Q22: At 20°C, a sample of gas has
Q23: A balloon filled with gas is heated
Q24: Which is a mathematical representation of Charles's
Q25: What happens to a helium balloon at