Not Answered
Use Bohr's model to draw a sodium (Na)atom and a chlorine (Cl)atom.Using your model,explain what happens when sodium reacts with chlorine to form table salt.Include in your explanation ion and ionic bond formation.Use your model to help you to decide whether NaCl is hydrophilic or hydrophobic. 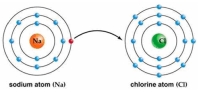
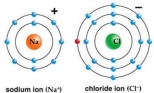
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Which of the following statements is/are true
Q9: Draw two hydrogen atoms using Bohr's model.Now
Q17: A change of one pH unit represents
Q21: Human blood has a pH of about
Q28: Classify the following substances as either hydrophobic
Q29: Study the figures to determine which is
Q38: The electrons are unequally shared in _,
Q49: In a water molecule,<br>A)the oxygen atom is
Q56: The scale indicates the relative concentrations of
Q57: As a solid, water floats. This means