Multiple Choice
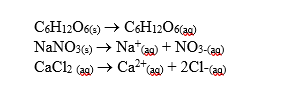
-Aqueous solutions with one mole of each solid in equal volumes of water are prepared.Which solution would have the lowest freezing point?
A) C6H12O6
B) NaNO3
C) CaCl2
D) All would have the same freezing point.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: According to the modern definition, NH<sub>3</sub> is<br>A)an
Q12: The water hardness in an area is
Q13: A solution is saturated when no more
Q14: What do solutions of acids, bases, and
Q15: Which of the following are properties of
Q17: Cooks add a pinch of salt to
Q18: Air is considered to be a homogeneous
Q19: When a solvent is cooled, it will
Q20: The solubility of a gas in water
Q21: What is the most likely temperature of