Multiple Choice
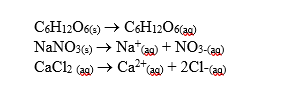
-One mole of particles lowers the freezing point of 1,000 g of water by 1.76ºC.What is the freezing point of a solution containing one mole of NaNO3 in 1,000 g of water?
A) 30.24ºC
B) 28.48ºC
C) -1.76ºC
D) -3.52ºC
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: The solubility of a gas in water
Q21: What is the most likely temperature of
Q22: The dye litmus turns blue in basic
Q23: A bottle of whiskey contains 40% alcohol
Q24: The rule "like dissolves like" explains why
Q25: Highway departments spread salt on icy roads
Q26: Polar compounds such as alcohol would be
Q28: If the force of attraction between the
Q29: In liquid solutions, the solute is<br>A)a solid.<br>B)a
Q30: Icebergs<br>A)have about the same density as sea