Multiple Choice
What is the formula when atoms of M and X (pictured below) react to form a stable ionic compound? 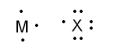
A) MX3
B) M3X
C) MX
D) MX5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: What type of chemical bond does the
Q15: The formation of an ionic bond<br>A)involves a
Q16: The representative elements have one to eight
Q17: Ionic compounds are generally<br>A)white, crystalline solids.<br>B)gaseous substances.<br>C)syrupy
Q18: Atoms gain or lose electrons in chemical
Q20: In an ionic compound, the metal<br>A)usually forms
Q21: The smallest unit of a covalent compound
Q22: When atoms of non-metallic elements react with
Q23: Atoms that have eight valence electrons would
Q24: What is the correct name for the