Multiple Choice
A photon is roughly 1800 times more massive than an electron. If a proton and an electron have the same kinetic energy,
A) the wavelength of the photon will be about 1800 times longer than the wavelength of the electron.
B) the wavelength of the photon will be about 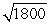 times longer than the wavelength of the electron.
times longer than the wavelength of the electron.
C) the wavelength of the photon will be roughly equal to the wavelength of the electron.
D) the wavelength of the electron will be about 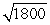 times longer than the wavelength of the photon.
times longer than the wavelength of the photon.
E) the wavelength of the electron will be about 1800 times longer than the wavelength of the photon.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Calculate the wavelength, in nanometers, of the
Q32: Calculate the frequency of the light emitted
Q63: A ground-state atom of iron has _
Q63: The electron in a hydrogen atom falls
Q87: Which one of the following sets of
Q88: Which ground-state atom has an electron configuration
Q101: An electron in a 3p orbital could
Q102: The electron configuration of a ground-state Co
Q115: A ground-state chromium atom has how many
Q122: A photovoltaic cell converts light into electrical