Multiple Choice
Which ground-state atom has an electron configuration described by the following orbital diagram? 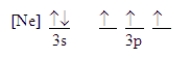
A) phosphorus
B) nitrogen
C) arsenic
D) vanadium
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: Calculate the wavelength of the light emitted
Q40: A neon atom in its ground state
Q42: What is the ground-state electron configuration for
Q50: If a hydrogen atom and a helium
Q58: The colors of the visible spectrum are
Q59: A ground-state atom of arsenic has<br>A)no unpaired
Q82: Write the ground state electron configuration for
Q84: Breaking the oxygen-oxygen bond in hydrogen peroxide
Q86: What is the electron configuration of calcium?
Q87: What is the wavelength, in meters, of