Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 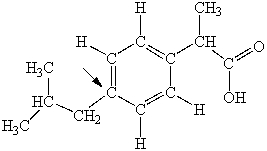
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The geometry of the CS<sub>2</sub> molecule is
Q11: According to the VSEPR theory, all of
Q21: Which of the following correctly lists species
Q51: Indicate the type of hybrid orbitals used
Q98: According to VSEPR theory, which one of
Q108: Consider the species O<sub>2</sub><sup>-</sup>, O<sub>2</sub>, and O<sub>2</sub><sup>+</sup>.
Q114: Use VSEPR theory to explain why the
Q116: Give the number of lone pairs around
Q122: According to VSEPR theory, which one of
Q134: What bond angles are predicted by VSEPR