Multiple Choice
Silver metal crystallizes in a face-centered cubic lattice with L as the length of one edge of the unit cube. The center-to-center distance between nearest silver atoms is
A) L/2.
B) 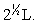
C) 2L.
D) 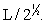
E) None of the above.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Suppose the atoms in a two-dimensional crystal
Q23: Which of the following would be expected
Q26: MgO has the same crystal structure as
Q44: Which of the following substances should have
Q56: The normal boiling point of bromine is
Q63: Iron crystallizes in a body-centered cubic unit.The
Q66: Of the given pair of compounds, which
Q70: The molar enthalpy of vaporization of carbon
Q100: Indicate all the types of intermolecular forces
Q100: Of the given pair of compounds, which