Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 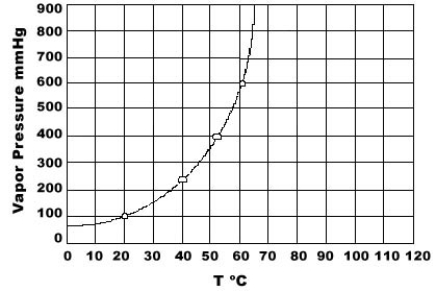
A) 19°C
B) 52°C
C) 60°C
D) 64°C
E) 70°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Given that the heat of vaporization of
Q13: Copper crystallizes in a face-centered cubic unit
Q59: Given the following liquids and their boiling
Q66: The triple point of iodine is at
Q88: What phase exists at the point labeled
Q91: Octane is a liquid component of gasoline.Given
Q94: Which of the following substances would have
Q114: The molar heats of sublimation and fusion
Q128: Which of the following liquids would have
Q140: Platinum has a face-centered cubic crystal structure