Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of O2. 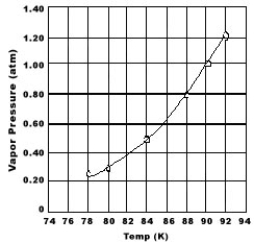
A) 92 K
B) 90 K
C) 88 K
D) 84 K
E) O2 doesn't boil because it is always a gas.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: Of the given pair of compounds, which
Q64: Potassium crystallizes in a body-centered cubic lattice.
Q66: The triple point of iodine is at
Q69: Find the temperature at which water boils
Q88: Which one of the following elements would
Q88: What phase exists at the point labeled
Q96: Which of the following properties indicates the
Q116: Find the temperature at which ethanol boils
Q128: Which of the following liquids would have
Q140: Platinum has a face-centered cubic crystal structure