Multiple Choice
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 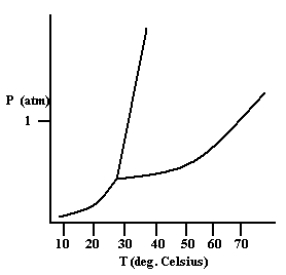
A) The melting point will decrease.
B) The melting point will remain the same.
C) The melting point will increase.
D) The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which of the following substances should have
Q56: Arrange the following in order of increasing
Q61: Which one of the following substances should
Q71: Calculate the amount of enthalpy required
Q79: Which one of the following substances crystallizes
Q90: Ethanol and dimethyl ether have the same
Q105: For which of the following species are
Q111: Indicate all the types of intermolecular forces
Q112: Boron nitride, BN<sub>3</sub>, melts at approximately at
Q146: Identify the dominant (strongest)type of intermolecular force