Multiple Choice
Which of the following elements would be expected to be particularly stable?
A) 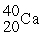
B) 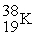
C) 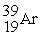
D) 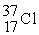
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: In which set do all elements tend
Q61: How many electrons are in the ion,CO<sub>3</sub><sup>2-</sup>?<br>A)16<br>B)28<br>C)30<br>D)32
Q148: A radioisotope which is neutron poor and
Q177: The charge-to-mass ratio of an electron was
Q221: Butyric acid has the structural formula given
Q222: Which of the compounds,Li<sub>3</sub>P,PH<sub>3</sub>,C<sub>2</sub>H<sub>6</sub>,IBr<sub>3</sub>,are ionic compounds?<br>A)only C<sub>2</sub>H<sub>6</sub><br>B)only
Q223: What are the names of the ions
Q224: What is the chemical formula for strontium
Q226: The term "nucleons" refers to the number
Q230: Tell the type of decay process occurring