Multiple Choice
Tell the type of decay process occurring in the following nuclear reaction. 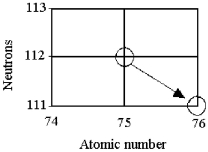
A) α emission
B) β emission
C) γ emission
D) electron capture or positron emission
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What is the charge on the Cr
Q27: Which is most likely to form a
Q41: Isotopes have the same number of _
Q93: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What kind of
Q95: The compound,Cu( I<sub> </sub>O<sub>3</sub> <sub> </sub>)<sub>2</sub>,is named<br>A)copper
Q96: Steel is galvanized by giving it a
Q103: The compound,Cu(ClO<sub>3</sub>)<sub>2</sub>,is named<br>A)copper chlorate(II).<br>B)copper(I)chlorate.<br>C)copper(I)chlorate(II).<br>D)copper(II)chlorate.
Q199: An element has two naturally occurring isotopes.One
Q212: How many protons (p)and neutrons (n)are in
Q236: The ions ClO<sub>4</sub><sup>-</sup>,ClO<sub>3</sub><sup>-</sup>,ClO<sub>2</sub><sup>-</sup>,and ClO<sup>-</sup> are named respectively<br>A)hypochlorate,chlorate,chlorite,perchlorite.<br>B)hypochlorite,chlorite,chlorate,perchlorate.<br>C)perchlorate,chlorate,chlorite,hypochlorite.<br>D)perchlorite,chlorite,chlorate,hypochlorate.