Multiple Choice
Use the periodic table below to answer the following questions. 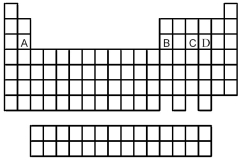
-Which is the correct formula of the binary fluoride of element A?
A) AF2
B) AF3
C) AF5
D) AF6
Correct Answer:

Verified
Correct Answer:
Verified
Q226: Which of the following statements concerning ionic
Q227: How many moles and how many atoms
Q228: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q229: The solid compound,Na<sub>2</sub>CO<sub>3</sub>,contains<br>A)Na<sup>+</sup>,C<sup>4+</sup>,and O<sup>2- </sup>ions.<br>B)Na<sup>+</sup> ions and
Q230: Which element indicated by letter in the
Q232: If unshaded spheres represent sulfur atoms and
Q233: Which of the following elements is a
Q234: What is the element symbol for an
Q235: What is the most likely charge on
Q236: The ions ClO<sub>4</sub><sup>-</sup>,ClO<sub>3</sub><sup>-</sup>,ClO<sub>2</sub><sup>-</sup>,and ClO<sup>-</sup> are named respectively<br>A)hypochlorate,chlorate,chlorite,perchlorite.<br>B)hypochlorite,chlorite,chlorate,perchlorate.<br>C)perchlorate,chlorate,chlorite,hypochlorite.<br>D)perchlorite,chlorite,chlorate,hypochlorate.