Multiple Choice
Which nuclide below is most likely to decay by electron capture?
A) 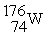
B) 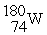
C) 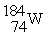
D) 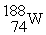
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of the following statements about positrons
Q76: The number of protons,neutrons,and total nucleons in
Q77: Which of the following represent isotopes?<br>A: <img
Q78: The isotope represented by <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt="The
Q80: Which is the only element that contains
Q82: The solid compound,Mg(NO<sub>3</sub>)<sub>2</sub>,contains<br>A)Mg<sub>2+</sub>,N5+,and O<sup>2-</sup> ions.<br>B)Mg<sub>2+</sub> ions and
Q83: The compound,SO<sub>3</sub>,is named<br>A)sulfate.<br>B)sulfite.<br>C)sulfur trioxide.<br>D)sulfur ( VI)oxide.
Q149: The nuclear transformation potassium-40 argon-40 + ?
Q154: What is the mass of one atom
Q223: Which is most likely to form a