Short Answer
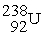
undergoes alpha decay producing one alpha particle and a single nuclide.To balance the equation,________ and ________ must be added to the right side of the equation below. 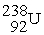
→ ? + ?
Correct Answer:

Verified
Correct Answer:
Verified
Q56: Which of the following represent isotopes?<br>[A: <img
Q57: The number of atoms of carbon in
Q58: Tell the type of decay process occurring
Q59: How many electrons are in the ion,PO<sub>4</sub><sup>3-</sup>?<br>A)26<br>B)44<br>C)47<br>D)50
Q62: Which reaction below represents <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt="Which
Q63: When a substance decays by beta emission,the
Q64: Identify the chemical symbol of element Q
Q65: Which one of the following compounds contains
Q80: Which one of the following compounds contains
Q120: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What kind of