Multiple Choice
If unshaded spheres represent nitrogen atoms and shaded spheres represent oxygen atoms,which box represents reactants and which represents products for the reaction 2 NO2(g) → 2 NO(g) + O2 (g) ? 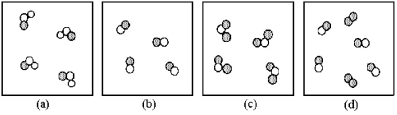
A) box (a) reactants and box (b) products
B) box (a) reactants and box (d) products
C) box (c) reactants and box (b) products
D) box (c) reactants and box (d) products
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Balance the chemical equation given below,and determine
Q7: How many milliliters of 0.550 M hydriodic
Q9: If 100.mL of 0.100 M Na<sub>2</sub>SO<sub>4</sub> is
Q11: What is the molar mass of 1-butene
Q12: Which of the following has the greatest
Q15: When 280.mL of 1.50 × 10<sup>-4 </sup>M
Q28: How many grams of the excess reagent
Q59: Which one of the following statements about
Q102: Combustion analysis of a 0.675 g sample
Q203: How many grams of AgNO<sub>3</sub> are needed