Multiple Choice
The following diagrams represent the reaction of A2 (shaded spheres) with B2 (unshaded spheres) .Identify the limiting reactant and write a balanced equation for the reaction. 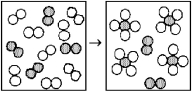
A) A2 is the limiting reactant;A + 4 B → AB4.
B) A2 is the limiting reactant;A2 + 4 B2 → 2 AB4.
C) B2 is the limiting reactant;A + 4 B → AB4.
D) B2 is the limiting reactant;A2 + 4 B2 → 2 AB4.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Which contains Avogadro's number of formula units?<br>A)36.5
Q25: What is the stoichiometric coefficient for oxygen
Q27: What is the concentration of an AlCl<sub>3</sub>
Q31: When 7.00 × 10<sup>22</sup> molecules of ammonia
Q32: Combustion analysis of 2.400 g of an
Q34: What is the concentration of FeCl<sub>3</sub> in
Q38: 5.0 g of iron is reacted with
Q60: How many moles of BCl<sub>3</sub> are needed
Q90: Which statement about elemental analysis by combustion
Q101: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Acetone has the