Multiple Choice
The following diagrams represent the reaction of A2 (shaded spheres) with B2 (unshaded spheres) .Identify the limiting reactant and write a balanced equation for the reaction. 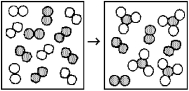
A) A2 is the limiting reactant;A + 3 B → AB3.
B) A2 is the limiting reactant;A2 + 3 B2 → 2 AB3.
C) B2 is the limiting reactant;A + 3 B → AB3.
D) B2 is the limiting reactant;A2 + 3 B2 → 2 AB3.
Correct Answer:

Verified
Correct Answer:
Verified
Q8: When 125 mL of 0.500 M AgNO<sub>3</sub>
Q36: How many grams of calcium chloride are
Q37: What is the molar mass of butane
Q38: 5.0 g of iron is reacted with
Q39: What is the balanced chemical equation for
Q40: What is the molar mass of hydrogen
Q42: How many milliliters of 0.200 M FeCl<sub>3</sub>
Q45: What is the empirical formula of a
Q57: Which one of the following contains 39%
Q130: What mass of phosphorus pentafluoride,PF<sub>5</sub>,has the same