Multiple Choice
Based on the positions in the periodic table of elements A,B,and C,which of the following reactions would you expect to occur? 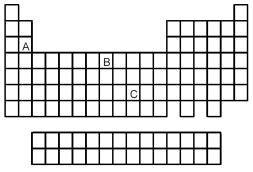
A) A2+ + B → A + B2+
B) B2+ + C → B + C2+
C) C2+ + A → C + A2+
D) None of the reactions would be expected to occur.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: The mixing of which pair of reactants
Q40: Write a balanced net ionic equation for
Q96: What are the coefficients in front of
Q97: Which of the following compounds is not
Q103: What is the molar concentration of sulfate
Q105: Write a balanced net ionic equation for
Q108: Which one of the following compounds behaves
Q115: When dissolved in water,KOH behaves as:<br>A)an acid
Q152: Write a net ionic equation for the
Q211: The acids HNO<sub>3</sub> and HNO<sub>2</sub> are named