Multiple Choice
Two electromagnetic waves are represented below. 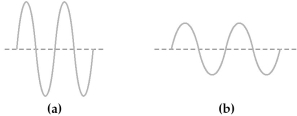
-Wave (b) has the
A) higher amplitude and greater intensity than wave (a) .
B) higher amplitude and weaker intensity than wave (a) .
C) lower amplitude and greater intensity than wave (a) .
D) lower amplitude and weaker intensity than wave (a) .
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Which of the following represent electron configurations
Q20: The subshell designations follow the alphabet after
Q21: What is the de Broglie wavelength of
Q22: The algebraic signs (+ and -)sometimes written
Q23: For a particular orbital,as one goes away
Q26: Which orbitals do not have a node
Q27: Which of the following elements would you
Q28: Rank the following elements in order of
Q29: Which of the following elements will have
Q75: For a hydrogen atom,which electronic transition would