Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 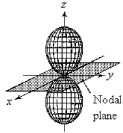
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:

Verified
Correct Answer:
Verified
Q17: A quantized variable<br>A)can be continuously varied.<br>B)can only
Q36: Molybdenum has an anomalous electron configuration.Write the
Q53: The amount of data that can be
Q60: What are the possible values of l
Q75: Copper has the anomalous electron configuration _.
Q81: Atoms of which element,indicated by letter on
Q88: The symbol [Kr] represents<br>A)4s<sup>2</sup>4p<sup>6</sup>.<br>B)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>4p<sup>6</sup>.<br>C)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</sup>4s<sup>2</sup>4p<sup>6</sup>.<br>D)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</sup>4s<sup>2</sup>4p<sup>6</sup>4d<sup>10</sup>.
Q106: Which of the following is true?<br>A)The Bohr
Q157: Which statement is false?<br>A)For any atom,the 4s
Q164: How many orbitals are there in the