Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 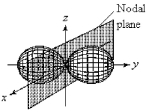
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:

Verified
Correct Answer:
Verified
Q11: The wave characteristics of a large,moving object,such
Q20: The ground-state electron configuration of the oxide
Q29: Which orbital-filling diagram violates the Pauli exclusion
Q49: Within a given shell of a multielectron
Q71: The spheres below represent atoms of Sb,As,P,and
Q82: What is the general valence-electron ground-state electron
Q92: A baseball with a mass of 150
Q118: Two electromagnetic waves are represented below. <img
Q128: Molecular vibrational energy transitions are observed in
Q141: What is the frequency of a helium-neon