Multiple Choice
Which period of elements,indicated by letter on the periodic table,has electrons whose highest principal quantum number n is 5? 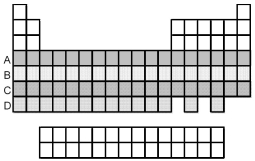
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: The property of a wave that is
Q20: The subshell designations follow the alphabet after
Q69: How many subshells are there in the
Q77: List all the elements that have a
Q102: Which of the following is true? The
Q109: Photochemists use electromagnetic radiation to initiate chemical
Q124: According to the Balmer-Rydberg equation,which transition results
Q128: A radio station that broadcasts at 99.5
Q129: Which orbitals do not have a node
Q130: Which of the following represent electron configurations