Multiple Choice
Which group of elements,indicated by letter on the periodic table,has electrons with the ground-state valence-shell electron configuration ns2 np4? 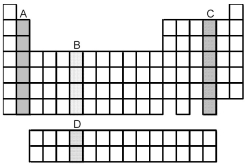
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: For an orbital,a node is<br>A)the midpoint of
Q75: Copper has the anomalous electron configuration _.
Q89: According to the Balmer-Rydberg equation,electromagnetic radiation with
Q92: Which orbital-filling diagram represents the anomalous ground
Q99: How many unpaired electrons are in an
Q112: The smallest number that can be used
Q119: What is the ground-state electron configuration of
Q120: The spheres below represent atoms of Sb,As,P,and
Q157: Which statement is false?<br>A)For any atom,the 4s
Q164: How many orbitals are there in the