Multiple Choice
Calculate the energy change for the formation of LiCl(s) from its elements in their standard states and the following tabulated information: 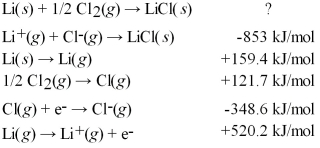
A) +1305.7 kJ/mol
B) +296.9 kJ/mol
C) -400.3 kJ/mol
D) -627.2 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q20: The group 4A element that always obeys
Q31: Of the following,which element has the highest
Q36: Lattice energy increases with _ cation and
Q57: The ion that has 28 protons and
Q84: Which element has the highest first electron
Q84: Which of the following atoms with the
Q92: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q98: What is the ground-state electron configuration of
Q165: Which ionization process requires the most energy?<br>A)P(g)→
Q170: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt=" -Atoms of which