Multiple Choice
Calculate the lattice energy for MgCl2(s) using a Born-Haber cycle and the following information: 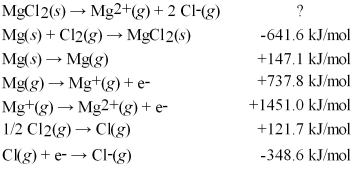
A) +641.6 kJ/mol
B) +1240.5 kJ/mol
C) +1882.1 kJ/mol
D) +2523.7 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q10: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q33: Using shorthand notation,the ground-state electron configuration for
Q62: Which of the following most likely represent
Q73: The element in period 3 with the
Q90: Each of the pictures (a)-(d)represents one of
Q90: What is the ground-state electron configuration of
Q100: Which chemical process is associated with the
Q108: Of the following,which element has the highest
Q112: Of the following,which element has the highest
Q116: Which contains ionic bonds?<br>A)CCl<sub>4</sub><br>B)CaCl<sub>2</sub><br>C)Cl<sub>2</sub><br>D)HCl