Multiple Choice
Calculate the energy change for the formation of CaF2(s) from its elements in their standard states and the following information: 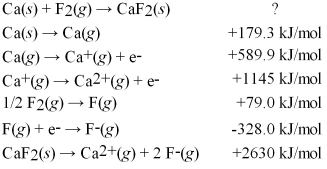
A) +4046 kJ/mol
B) -965 kJ/mol
C) -1214 kJ/mol
D) -3286 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Which period 2 element has successive first
Q59: List the elements Cs,Ca,Ne,Na,Ar in order of
Q66: The neutral atom with the electron configuration
Q68: Which liberates the most energy?<br>A)Br(g)+ e⁻ →
Q116: Which contains ionic bonds?<br>A)CCl<sub>4</sub><br>B)CaCl<sub>2</sub><br>C)Cl<sub>2</sub><br>D)HCl
Q129: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q137: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q147: Each of the pictures (a)-(d)represents one of
Q249: The product of the reaction of lithium
Q294: Which of the following elements is a