Multiple Choice
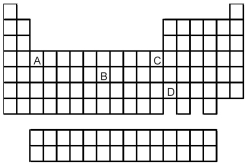
-Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration 1s2 2s2 2p6 3s2 3p6?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q43: Which ionization process requires the most energy?<br>A)O(g)→
Q52: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q54: Which is not generally considered to be
Q58: The following pictures represent alkali halide salts.
Q66: Which species does not have an octet
Q68: Which of the following ionic compounds would
Q71: Which contains covalent bonds?<br>A)NaH and HCl<br>B)only HCl<br>C)only
Q72: Which ionic compound would be expected to
Q121: Of the following,which element has the highest
Q136: The four spheres below represent Na<sup>+</sup>,Mg<sup>2+</sup>,F⁻,and O<sup>2-</sup>,not