Multiple Choice
The following four spheres represent a metal atom,a nonmetal atom,a monatomic anion and a monatomic cation,not necessarily in that order. 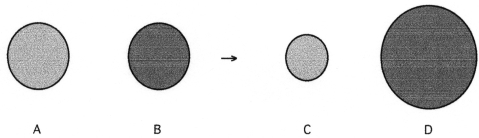
-Which sphere represents the monatomic anion?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Consider Li<sup>+</sup>,F<sup>-</sup>,and O<sup>2-</sup>.Which ratio should be the
Q12: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q13: How many electrons are in the outermost
Q14: Of the following,which element has the highest
Q15: The ion Q<sup>2+</sup> contains 36 electrons.The identity
Q17: Element A has a valence shell configuration
Q18: List the elements Na,Ca,Rb,Cl,He in order of
Q19: Which period 2 element has successive first
Q20: The group 4A element that always obeys
Q21: Calculate the energy change for the formation