Multiple Choice
The following four spheres represent a metal atom,a nonmetal atom,a monatomic anion and a monatomic cation,not necessarily in that order. 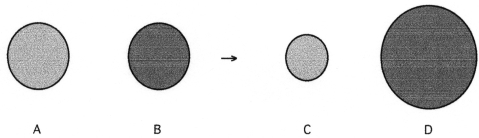
-Which sphere represents the monatomic cation?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q38: Which element has the most favorable (most
Q39: An element that has the valence electron
Q40: Which ionic compound would be expected to
Q41: How many valence shell electrons does an
Q42: Using shorthand notation,the ground-state electron configuration for
Q44: The four spheres below represent Na<sup>+</sup>,Mg<sup>2+</sup>,F⁻,and O<sup>2-</sup>,not
Q45: Which ionization process requires the most energy?<br>A)Pt(g)→
Q46: Which ionic compound would be expected to
Q47: The number of electrons in the ion
Q48: Which ionic compound would be expected to