Multiple Choice
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 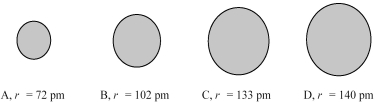
-Which sphere most likely represents the F- ion?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q128: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q129: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q130: Of the following,which element has the highest
Q131: The following four spheres represent a Na
Q132: Of the following,which element has the highest
Q133: Of the following,which element has the highest
Q134: Which liberates the most energy?<br>A)Li(g)+ e<sup>-</sup> →
Q135: Which element,indicated by letter on the periodic
Q137: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q138: Calculate the lattice energy for MgCl<sub>2</sub>(s)using a