Multiple Choice
Atoms of which element,indicated by letter on the periodic table,would be expected to have the most negative value of Eea? 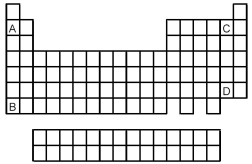
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: The following four spheres represent a metal
Q30: Indicate which is larger in each of
Q32: Which ion does not have a noble
Q35: The number of electrons in the ion
Q38: How many electrons are in the outermost
Q65: Which electron affinity process would liberate the
Q71: Isoelectronic means having the same number of
Q162: If niobium loses all of its valence
Q173: When the equation for the reaction of
Q243: Metals tend to react with the halogens