Multiple Choice
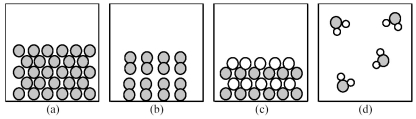
-Which of the above pictures best represents a solid ionic compound?
A) picture (a)
B) picture (b)
C) picture (c)
D) picture (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Which species does not have an octet
Q24: The element in period 4 with the
Q25: Which ionization process requires the most energy?<br>A)S(g)→
Q26: Which of the following ionic compounds would
Q27: Which of these elements has the most
Q29: Consider the following electron configurations for neutral
Q30: Which of the following ionic compounds would
Q31: The four spheres below represent K<sup>+</sup>,Ca<sup>2+</sup>,Cl<sup>-</sup>,and S<sup>2-</sup>,not
Q32: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q33: Using shorthand notation,the ground-state electron configuration for